Plant health can be affected by many influences. At The University of Guelph, Agriculture & Food Laboratory, with our experienced plant pathologists, we routinely provide scientific diagnostic results to resolve your plant health issues. Preventative measures are often much less expensive than curative approaches, while still impeding or avoiding disease development in plants as well as reducing or eliminating losses in quality. Our plant analyses encompass a wide variety of greenhouse vegetables, field crops and flowers.
The quality of crops can be affected by many things. Receive testing today and use our plant diagnostic services and standard/custom analytical packages.
Learn more below about our plant testing services or contact us to learn how our laboratories can meet your testing needs.
PLEASE NOTE: Due to import restrictions, we do not accept plant samples from outside Canada.
Nutrient Analysis
| Plant Package 2 | Total N, P, K, Mg, Ca, Al, B, Cu, Fe, Mn, Mo, Na, S and Zn in plant tissue |
| Total Nitrogen | Combustion Method |
| Total Carbon | Combustion Method |
| Plant Ammonium and Nitrate | KCl extractable NH4-N and NO3-N |
Plant Disease Diagnostics
Bacteria
Plant pathogenic bacteria are spread in many different ways and enter host plants through wounds or natural openings. They can cause severe diseases on plants including leaf and fruit spots, stem, fruit and tuber rots, blights, cankers, galls, scabs, and wilts.
Our experienced diagnosticians use disease symptoms, bacterial streaming, and/or plating on microbiological media to confirm bacterial infection in plant samples submitted to the clinic. Biolog or DNA sequencing is then used to identify the bacteria to species. Our rapid PCR methods can be used for detection of bacterial pathogens.
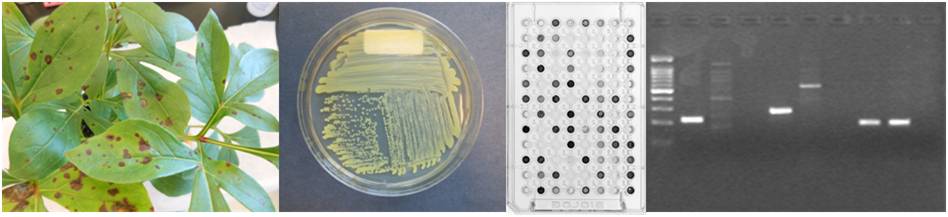
Biolog:
- Erwinia amylovora (Fire blight)
- Erwinia carotovora
- Pseudomonas syringae
- Ralstonia solanacearum (Tomato bacterial wilt)
- Xanthomonas campestris
- and many more
PCR detection is available for:
- Agrobacterium tumefaciens spp. (Ti and Ri plamids)
- Agrobacterium vitis (Crown gall of grapevine)
- Candidatus Liberibacter solanacearum
- Clavibacter michiganensis ssp. michiganensis (Tomato bacterial canker) (accredited by Standards Council of Canada)
- Dickeya spp. (Potato soft rot etc.)
- Erwinia amylovora (Fire blight)
- Erwinia tracheiphila (Bacterial wilt of cucurbit)
- Phytoplasmas including Aster Yellows
- Streptomyces spp. (Potato common scab)
- Xanthomonas spp. (Bacterial spot on tomato and sweet pepper)
- Xylella fastidiosa
Plant Disease Clinic Shipping & Sampling Guidelines
Fungi
Many plant diseases are caused by fungi and fungi-like organisms. Plant pathogenic fungi cause a very wide range of diseases including crown, fruit, root, stem, and tuber rots, leaf and fruit spots, blights, cankers, damping-off, diebacks, galls, rusts, and wilts.

Traditionally, fungal diseases have been diagnosed by disease symptoms and by observing fungal structures under a microscope. Structures such as hypae, spores, and the cells that produced spores must be examined to identify the fungal pathogen. When fungal structures are not present, symptomatic plant tissue can be placed in a moist chamber to promote fungal growth or can be plated on selective microbiological media to isolate the fungus. Our diagnosticians are experienced in traditional techniques for identification of fungal plant pathogens and combine these with the most modern DNA-based pathogen detection and identification techniques.

DNA Multiscan is an accurate and rapid test that is available for detection and identification of fungi. This test is offered in Canada exclusively by The University of Guelph, Agriculture & Food Laboratory (AFL) and is based on proven molecular technology protocols. The DNA Multiscan can be used to test for plant pathogenic fungi in plants, soil and water. The basic scan can accurately detect over 25 pathogens. The diagnostic scan, can accurately detect over 65 pathogens. Scans specific to turfgrass and ginseng are also offered. Results are available in 4-5 business days. Use the links below to see sample reports listing the fungi detected by the different DNA scans.
Sample Reports
- Basic Scan (Plant, water or soil)
- Diagnostic Scan (plant, water or soil)
- Turf Scan
PCR detection methods for fungal pathogens include:
- Aphanomyces cochlioides
- Aphanomyces euteiches
- Ceratocystis fagacearum (Oak wilt)
- Cylindrocarpon destructans f. sp. panacis (syn: Ilyonectria mors-panacis)
- Cylindrocladium buxicola (Boxwood blight)
- Fusarium oxysporum f. sp. lycopersici and F. oxysporum f. sp. radicis-lycopersici
- Pseudoperonospora humili (Hop Systemic downy mildew infection)
- Plasmodiophora brassicae (Club root)
- Pyrenochaeta lycopersici (Corky root)
- Spongospora subterranea f. sp. subterranea (Potato powdery scab)
- Verticillium spp. (V. dahlia, V. longisporum, V. alfalfae, V. albo-atrum and V. nonalfalfae)
Plant Disease Clinic Shipping & Sampling Guidelines
Viruses and Virus-Like Agents
Viruses and virus-like agents are the smallest of the plant pathogens and cannot be seen with a light microscope. They are non-cellular and unable to reproduce without the cellular machinery of their host. They are immobile and unable to disseminate without the aid of other organisms or the environment. The genetic material of most plant viruses is RNA but in some viruses it is DNA. Viruses cause a variety of symptoms in plants including curling, mosaic patterns, mottling, necrosis, puckering, ringspots, stunting, twisting, and wilting. Viruses are often named based on the host plant in which they were first identified and the symptoms they caused in that host. Symptoms caused by a virus can vary depending on host, age of host, and environmental conditions. Some virus symptoms can be very similar to those of nutrient deficiencies and environmental stresses.
Our diagnosticians recognize symptoms consistent with virus infection and use fast, reliable detection methods to test for the presence of common viruses.

The Plant Disease Clinic provides detection of viruses and virus-like agents in plants by ELISA (immunoassay), RT-PCR (RNA viruses) and PCR (DNA viruses).
ELISA (accredited by Standards Council of Canada)
We offer ELISA virus screens for the crops listed below. Virus screens test the common viruses of a crop. Please click below on the specific crop to view common viruses. We can also customize tests according to our client's requirements.
- See a list of viruses tested by ELISA method
Crop Specific Screens
- Barley/wheat screen
- Bean screen
- Blueberry/cranberry screen
- Cannabis screen *(Please contact us prior to submitting samples for this test)
- Corn screen
- Cucurbits screen
- Kalanchoe screen
- Orchid screen
- Ornamental screen
- Pepper screen
- Petunia screen
- Raspberry screen
- Soybean screen
- Strawberry screen
- Tomato screen
PCR/RT-PCR
Our PCR/RT-PCR detection methods include:
- Beet pseudo yellow virus (BPYV)
- Blackberry yellow vein associated virus (BYVaV)
- Carlavirus group
- Cucumber green mottle mosaic virus (CGMMV)
- Dahlia mosaic virus (DMV)
- Grapevine red blotch associated virus (GRBaV)
- Hop mosaic virus (HpMV)
- Hop latent virus (HpLV)
- Hop latent virus (HLVd)
- Hop stunt viroid (HSVd)
- IIarvirus Group
- Pepino mosaic virus (PepMV) and genotyping
- Pospiviroid
- Potyvirus group
- Raspberry left mottle virus (RLMV)
- Rubus yellow net virus (RYNV)
- Rose rosette virus
- Strawberry crinkle virus (SCV)
- Strawberry mottle virus (SMoV)
- Strawberry mild yellow edge virus (SMYEV)
- Strawberry pallidosis virus (SPaV)
- Strawberry vein banding virus (SVBV)
- Soybean vein necrosis virus (SVNV)
- Tobacco Rattle Virus (TRV)
- Tobamovirus group
- Tomato brown rugose fruit virus (ToBRFV)
- Tospovirus group
Plant Disease Clinic Shipping & Sampling Guidelines
Nematodes
Nematodes are microscopic roundworms that inhabit a wide range of environments. Plant-parasitic nematodes cause damage by feeding on the roots of host plants and, in certain cases, cause damage by feeding on above-ground parts of host plants. Nematode feeding causes reduction of root mass and distortion and/or enlargement of roots. Damage to the root system also allows infection by other soil-plant pathogens.
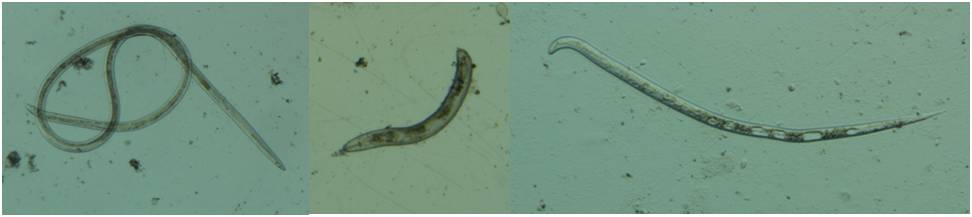
- Nematode Identification and Count from Roots/Plant Tissues
- Pinewood Nematode Detection in Wood
- Nematode Count from Soil (accredited by Standards Council of Canada)
- Cyst Nematode Count (accredited by Standards Council of Canada)
Plant Disease Clinic Shipping & Sampling Guidelines
Insect Identification
Insect pests can cause significant damage to host plants. The insect pests can be identified by DNA barcoding.

Plant Disease Clinic Shipping & Sampling Guidelines
Plant Identification
If you are looking for plant identification services, we recommend contacting the Royal Botanical Gardens in Burlington.
Apple Scab Fungicide Resistance Testing
The Plant Disease Clinic (PDC) can test for resistance to Syllit (Dodine), Nova (Myclobutanil), Inspire (Difenoconazole), and Flint (Trifloxystrobin) using a fungicide plating technique. You can choose to test for resistance to all of these fungicides or test one, two or three. There are leaf collection and testing suggestions below for ‘Just Checking’ and for ‘Suspected Control Failure’. Due to the nature of the testing used, results will take up to four months.
We also offer PCR testing for the G143A mutation, which is the cause of Trifloxystrobin resistance in most isolates. Results of G143A mutation PCR testing take approximately five business days.
Collection of leaves with apple scab is the most important step in fungicide resistance testing. If leaves have been sprayed, collected at the wrong time, or stored incorrectly, PDC may not be able to recover viable spores for testing. The success of fungicide resistance testing is greatest when leaves are submitted before the end of June.
Please follow the collection instructions below carefully.
I. Just Checking
Select six to eight test trees at the corner of a typical orchard block and leave them unsprayed until scab lesions develop on cluster leaves. When scab lesions develop on cluster leaves, collect 50 young leaves containing fresh lesions. After you have collected leaves for resistance testing, you are free to spray the test trees. Testing 12 or 25 isolates of the apple scab fungus from the test trees is recommended.
II. Suspected Control Failure
Collect 75-100 leaves with apple scab from throughout the orchard. Testing 25-50 isolates of the apple scab fungus is recommended. Collect leaves with fresh lesions.
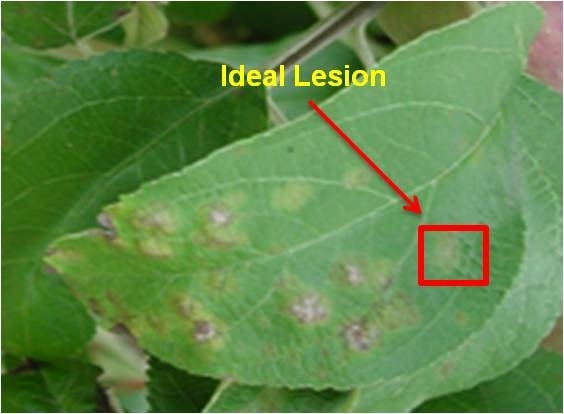



Photos courtesy of Kerik Cox, Cornell University.
III. Collection Instructions
PLEASE READ CAREFULLY!
- Collecting leaves with several separate scab lesions is OK but do not collect leaves with sheet scab.
- Please separate leaves with a paper towel or newspaper to absorb moisture. Collect leaves after the dew has dried.
- Do not collect leaves during or in the days after a hot spell (several days >28°C). Prolonged hot temperatures will kill the spores.
- Do not collect leaves immediately after a rain event. If there is a heavy or prolonged rain event, wait at least 48 hours after the rain stops before collecting leaves. Rain will wash spores from the leaves.
- After collecting leaves, keep them at temperatures lower than 24°C. Spores of the apple scab pathogen are more sensitive to heat than other pathogens and being left in a hot location, such as a vehicle in the sun, will kill the spores.
- Send leaves by overnight courier or drop them off at our clinic. Do not ship on a Friday. If necessary, leaves can be refrigerated for one or two days before shipping. Shipping leaves in a cooler with an ice pack is recommended, especially in hot weather.
IV. Sample Submission
Fill in a sample submission form to include with your shipment of leaves. Remember to indicate the fungicides to be tested as well as the number of isolates to be tested on the submission form.
Ship to:
- Plant Disease Clinic
University of Guelph
95 Stone Road West
Guelph ON N1G 2Z4
Tel: 519-823-1268 Ext. 57256
PCR Testing: PCR testing for the G143A mutation, which is responsible for Trifloxystrobin resistance in most isolates, is also available. Please contact us.
*The fungicide plating technique was developed with the support of the Pest Management Centre, Agriculture and Agri-Food Canada, and the Ontario Apple Growers.
Molecular Testing
Plant Species ID Determination
Our DNA-based plant ID test determines species of unknown plant materials such as vegetables, fruits, grains, and trees. The plant materials can be raw and sometimes processed or cooked.
Plant Variety/Cultivar Testing
There are many varieties/cultivars of a particular plant species. DNA-based tests accurately identify varieties/cultivars to ensure “true-to-variety”. This testing can confirm the presence of a superior trait, character, or value, for exporting acceptance, or for regulatory compliance. For example, there are six varieties/cultivars of Tebou beans and four types of Azuki beans that look alike.
Only certain varieties/cultivars are acceptable for exporting purposes or having superior quality (value). Our DNA tests provide identification/discrimination, as well as QC-check (for contamination of un-wanted varieties/cultivars) among the Azuki and Tebou bean varieties/cultivars. Our variety/cultivar testing includes, but is not limited to:
- Apples (e.g. Golden Delicious & McIntosh)
- Azuki beans (e.g. Erimosyouzu & Kitanootome)
- Potatoes (e.g. Yukon Gold)
- Raspberries
- Strawberries
- Tebou beans (e.g. Hemi-Tebou & Yuki-Tebou)
- Tobacco (e.g. distinguishing Canadian from American varieties)
Pesticide Testing
AFL has both single residue and multi-residue capabilities to test over 500 pesticide active ingredients. Our testing services allow you to address a wide range of concerns pertaining to the use and application of pesticides on all crops, vegetables, fruits, grains, and other vegetation.
Other Useful Links:


